In which one of the following arrangements the given sequence is not strictly according to the properties indicated against it
In which one of the following arrangements the given sequence is not strictly according to the properties indicated against it
CO2 < SiO2 < SnO2 < PbO2 :Increasing oxidizing power
HF < HCl< HBr < HI:Increasing acidic strength
H2O < H2S< H2Se < H2Te:Increasing pKa values
NH3 < PH3< AsH3 < SbH3:Increasing acidic character
The Correct Option is C
Solution and Explanation
The question asks which among the given options does not strictly follow the sequence according to the indicated property. Let's analyze each option to determine the correctness:
-
CO2 < SiO2 < SnO2 < PbO2: Increasing oxidizing power
This sequence is based on the oxidizing power of the oxides from Group 14 elements. As we move down the group from CO2 to PbO2, the oxidizing power generally increases. Therefore, this sequence is correct.
-
HF < HCl < HBr < HI: Increasing acidic strength
In binary acids of halogens, the acidic strength increases from HF to HI due to the increase in bond length and decrease in bond dissociation energy. This makes HI the strongest acid, and the sequence is correct.
-
H2O < H2S < H2Se < H2Te: Increasing pKa values
The statement implies that the pKa value increases, suggesting a decrease in acidic strength. However, in reality, H2Te is a stronger acid than H2O, meaning the pKa would decrease down the group. Hence, this sequence is incorrect for increasing pKa values.
-
NH3 < PH3 < AsH3 < SbH3: Increasing acidic character
As we move down Group 15 hydrides, the acidic character increases due to the decrease in bond energy and increase in atomic size, which makes the bond weaker. Thus, this sequence is correct.
Conclusion: The sequence H2O < H2S < H2Se < H2Te: Increasing pKa values does not correctly follow the properties indicated since pKa values should decrease with the increase in acidic strength. Therefore, the correct answer is this option.
Top Questions on p -Block Elements
Method used for separation of mixture of products (B and C) obtained in the following reaction is:

- JEE Main - 2026
- Chemistry
- p -Block Elements
- Statement-I: An element ‘X’ of P-block forms a hydride H–X, which has the longest bond length, then element ‘X’ will have the shortest covalent radius.
Statement-II: An element ‘E’ of Group 15 forms hydride EH$_3$, that has least B.P. The maximum covalency of E is 4.
- JEE Main - 2026
- Chemistry
- p -Block Elements
- Given below are two statements:
Statement I: [CoBr₄]²⁻ ion will absorb light of lower energy than [CoCl₄]²⁻ ion.
Statement II: In [CoBr₄]²⁻ ion, the energy separation between the two set of d-orbitals is more than [CoCl₄]²⁻ ion.
In the light of the above statements, choose the correct answer from the options given below :- JEE Main - 2026
- Chemistry
- p -Block Elements
- Given below are two statements: Statement I: Elements \(X\) and \(Y\) are the most and least electronegative elements, respectively, among \(N\), \(As\), \(Sb\) and \(P\). The nature of the oxides \(X_2O_3\) and \(Y_2O_3\) is acidic and amphoteric, respectively. Statement II: \(BCl_3\) is covalent in nature and gets hydrolysed in water. It produces \([B(OH)_4]^-\) and \([B(H_2O)_6]^{3+}\) in aqueous medium. In the light of the above statements, choose the correct answer from the options given below:
- JEE Main - 2026
- Chemistry
- p -Block Elements
- For \( P \), the incorrect statement is:
- JEE Main - 2026
- Chemistry
- p -Block Elements
Questions Asked in NEET exam
- Two cities X and Y are connected by a regular bus service with a bus leaving in either direction every T min. A girl is driving scooty with a speed of 60 km/h in the direction X to Y. She notices that a bus goes past her every 30 minutes in the direction of her motion, and every 10 minutes in the opposite direction. Choose the correct option for the period T of the bus service and the speed (assumed constant) of the buses.
- NEET (UG) - 2025
- Relative Velocity
- A physical quantity P is related to four observations a, b, c, and d as follows: P = a3 b2 (c / √d) The percentage errors of measurement in a, b, c, and d are 1%, 3%, 2%, and 4% respectively. The percentage error in the quantity P is:
- NEET (UG) - 2025
- Dimensional analysis and its applications
What is Microalbuminuria ?
- NEET (UG) - 2025
- Human physiology
The output (Y) of the given logic implementation is similar to the output of an/a …………. gate.

- NEET (UG) - 2025
- Logic gates
- An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature \(27^\circ\)C. The mass of the oxygen withdrawn from the cylinder is nearly equal to: [Given, \(R = \frac{100}{12} \text{ J mol}^{-1} \text{K}^{-1}\), and molecular mass of \(O_2 = 32 \text{ g/mol}\), 1 atm pressure = \(1.01 \times 10^5 \text{ N/m}^2\)]
- NEET (UG) - 2025
- Ideal-gas equation and absolute temperature
Concepts Used:
Group 16 Elements
The group 16 elements (oxygen group elements) of the periodic classification are also known as chalcogens because most of the copper ores have copper in the form of oxides and sulphides. The word chalcogen means “ore formation” which is derived from the Greek word “Chalcos” (Ore) and “gen” (formation).
There are 5 elements that come under Group 16 of the Modern Periodic Table namely:
- Oxygen (O)
- Sulphur (S)
- Selenium (Se)
- Tellurium (Te)
- Polonium (PO)
Electronic Configuration:
The general electronic configuration of the chalcogens can be written as ‘ns2np4’, where ‘n’ denotes the value of the principal quantum number corresponding to the valence shell of the element.
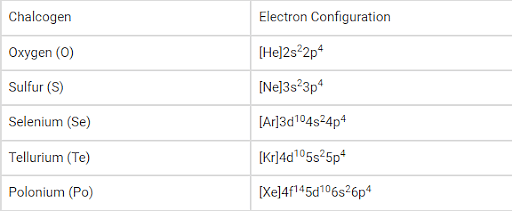
The electron configuration of the synthetic element livermorium (believed to be a chalcogen) is predicted to be [Rn]5f146d107s27p4.
Metallic Nature of the Group 16 Elements:
- Oxygen and sulfur are classified as non-metals.
- Selenium and tellurium are classified as metalloids.
- Under standard conditions, polonium exhibits metallic characteristics. However, it is important to note that polonium is a radioactive element.