In Haber process $30\,L$ of dihydrogen and $30\,L$ of dinitrogen were taken for reaction which yielded only $50\%$ of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end?
- 20 L ammonia, 10 L nitrogen, 30 L hydrogen
- 20 L ammonia, 25 L nitrogen, 15 L hydrogen
- 20 L ammonia, 20 L nitrogen, 20 L hydrogen
- 10 L ammonia, 25 L nitrogen, 15 L hydrogen
The Correct Option is D
Approach Solution - 1
The correct answer is Option D) 10 L ammonia, 25 L nitrogen, 15 L hydrogen
In Haber process 30L of dihydrogen and 30L dinitrogen were taken for reaction which yielded only 50% of the expected product.
The Haber process is the technique used to make ammonia. Ammonia is created when hydrogen and nitrogen react. Extremely exothermic in nature, this reaction produces heat as it proceeds. The result is
\(N_2 + 3H_2 \rightarrow 2NH_3\)
\(_{10L}^{1V} \, \, \, \, _{30L}^{3V} \, \, \, \, \, _{20L}^{2V}\)
Two moles of ammonia are produced when one mole of nitrogen combines with three moles of hydrogen.
As only 50% of the expected product is formed,
hence only 10 L of \(NH_3\) is formed.
the composition of gaseous mixture under the aforesaid condition in the end will be-
\(H_2 = 30 - 15 = 15 \, L\)
\(N_2 = 30 - 5 = 25 \, L\)
\(NH_3 = 10 \, L\)
If the reaction contains a coefficient, we must subtract the assumed quantity multiplied by the reactant's coefficient from the supplied amount of the reactant to get the amount of reactant that is still present in the reaction.
Discover more from this chapter: Equilibrium
Approach Solution -2
The correct answer is Option D) 10 L ammonia, 25 L nitrogen, 15 L hydrogen
Real Life Applications
- The Haber process is a real-life application of the equilibrium concept in chemistry.
- The Haber process is a very important industrial process, and it is used to produce a variety of products that are essential to our lives.
- The real-life application of the Haber process enlightens us on how chemistry can be used to solve practical problems and ease our lives.
Question can also be asked as
- What is the composition of the gaseous mixture after the Haber process has yielded 50% of the expected product?
- How much dihydrogen, dinitrogen, and ammonia will be present in the gaseous mixture after the Haber process has yielded 50% of the expected product?
- What is the volume of each gas in the gaseous mixture after the Haber process has yielded 50% of the expected product?
Approach Solution -3
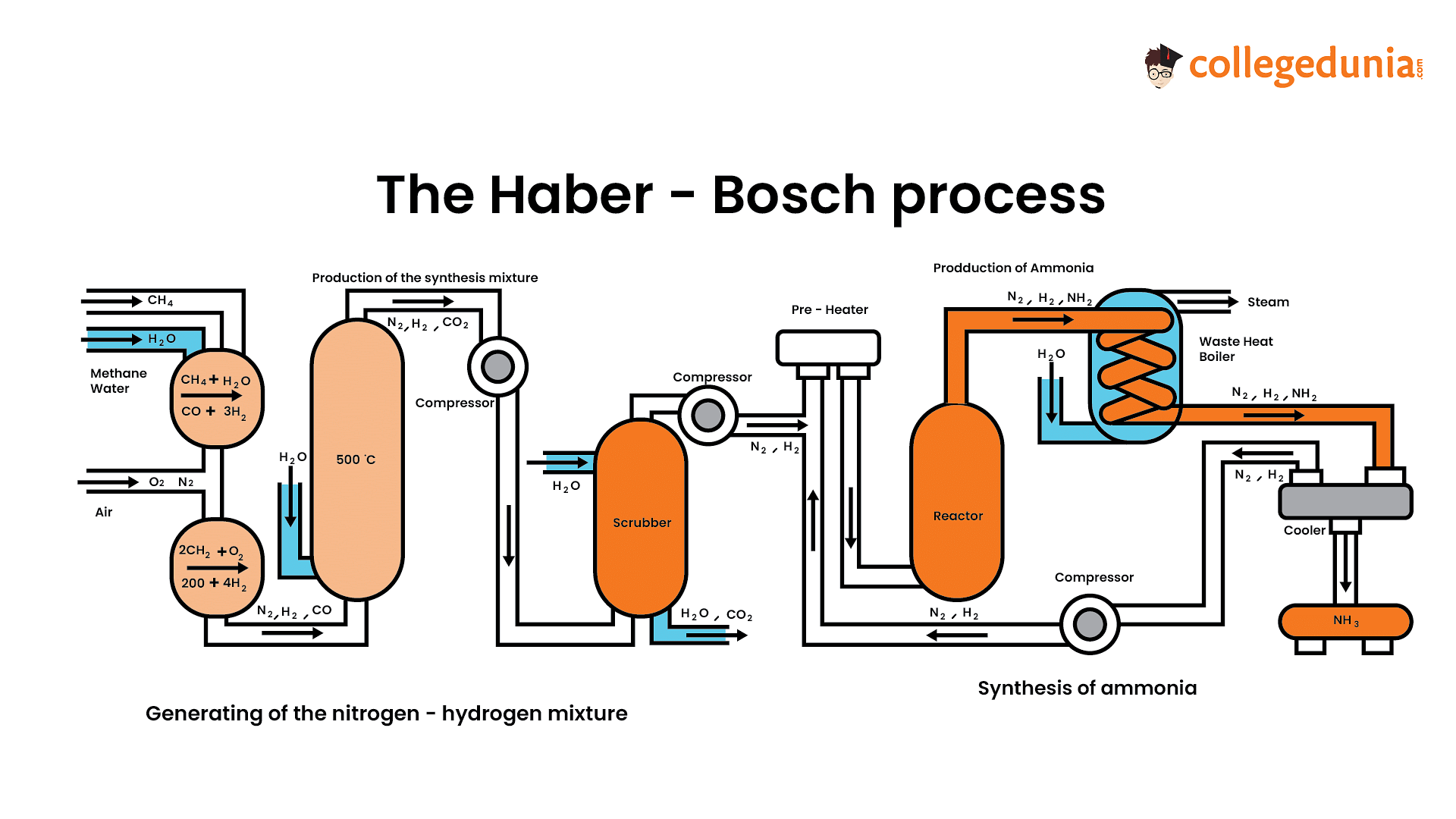
The Haber Process, also known as the Haber-Bosch Process, is one of the most economically and effectively produced industrial ammonia processes. Throughout the 20th century, a German scientist named Fritz Haber and his assistant developed the Haber process catalyst and high-pressure machinery to carry out this process in a lab. In the year 1910, Carl Bosch took the idea and developed it into a tool for industrial manufacturing. This was a tremendous scientific achievement.
Raw Materials Used in the Haber Process
A great illustration of how industrial chemists make use of the factors that affect chemical equilibria is the Haber-Bosch process. This is used to identify the ideal circumstances for creating a lot of goods at a fair price.
- The Haber-Bosch reaction turns nitrogen gas into ammonia by combining it with hydrogen gas.
- This procedure, which is performed at high temperatures and pressures, makes use of a metal catalyst.
The process is as follows:
- Air is used to supply nitrogen.
- Natural gas and water provide hydrogen as well as the heat needed to heat the reactants.
- During the process, the catalyst, iron, is not used up.
Haber Process
In the Haber Bosch method, hydrogen atoms obtained from natural gas are mixed in a 1:3 volume ratio with nitrogen gas received from the air.
- The gases are cycled, each pass cooling them, across four catalyst beds.
- This procedure keeps the balance in place.
- Unreacted gases are recycled at various conversion criteria for each run.
- Most frequently, the procedure makes use of an iron catalyst.
- It takes place between 150 and 200 atmospheres of pressure and 400 to 450 degrees Celsius in temperature.
- This process includes the removal of carbon dioxide, shift conversion, methanation, and steam reforming.
- At the conclusion of the procedure, the liquid solution created by cooling the ammonia gas is collected and maintained in storage containers.
Top Questions on Equilibrium
Consider the following equilibrium,
CO(g) + 2H2(g) ↔ CH3OH(g)
0.1 mol of CO along with a catalyst is present in a 2 dm3 flask maintained at 500 K. Hydrogen is introduced into the flask until the pressure is 5 bar and 0.04 mol of CH3OH is formed. The Kp is ____ × 10-3 (nearest integer).
Given: R = 0.08 dm3 bar K-1mol-1
Assume only methanol is formed as the product and the system follows ideal gas behaviour.
- JEE Main - 2025
- Chemistry
- Equilibrium
The pH of a 0.01 M weak acid $\mathrm{HX}\left(\mathrm{K}_{\mathrm{a}}=4 \times 10^{-10}\right)$ is found to be 5 . Now the acid solution is diluted with excess of water so that the pH of the solution changes to 6 . The new concentration of the diluted weak acid is given as $\mathrm{x} \times 10^{-4} \mathrm{M}$. The value of x is _______ (nearest integer).
- JEE Main - 2025
- Chemistry
- Equilibrium
A body of mass $m$ is suspended by two strings making angles $\theta_{1}$ and $\theta_{2}$ with the horizontal ceiling with tensions $\mathrm{T}_{1}$ and $\mathrm{T}_{2}$ simultaneously. $\mathrm{T}_{1}$ and $\mathrm{T}_{2}$ are related by $\mathrm{T}_{1}=\sqrt{3} \mathrm{~T}_{2}$. the angles $\theta_{1}$ and $\theta_{2}$ are
- JEE Main - 2025
- Physics
- Equilibrium
- According to Le Chatelier's principle, in the reaction \( \text{CO}(g) + 3\text{H}_2(g) \rightleftharpoons \text{CH}_4(g) + \text{H}_2\text{O}(g) \), the formation of methane is favoured by
- KCET - 2025
- Chemistry
- Equilibrium
- Which of the following statements is/are true about equilibrium?
- KCET - 2025
- Chemistry
- Equilibrium
Questions Asked in NEET exam
- Two cities X and Y are connected by a regular bus service with a bus leaving in either direction every T min. A girl is driving scooty with a speed of 60 km/h in the direction X to Y. She notices that a bus goes past her every 30 minutes in the direction of her motion, and every 10 minutes in the opposite direction. Choose the correct option for the period T of the bus service and the speed (assumed constant) of the buses.
- NEET (UG) - 2025
- Relative Velocity
- A physical quantity P is related to four observations a, b, c, and d as follows: P = a3 b2 (c / √d) The percentage errors of measurement in a, b, c, and d are 1%, 3%, 2%, and 4% respectively. The percentage error in the quantity P is:
- NEET (UG) - 2025
- Dimensional analysis and its applications
What is Microalbuminuria ?
- NEET (UG) - 2025
- Human physiology
The output (Y) of the given logic implementation is similar to the output of an/a …………. gate.

- NEET (UG) - 2025
- Logic gates
- An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature \(27^\circ\)C. The mass of the oxygen withdrawn from the cylinder is nearly equal to: [Given, \(R = \frac{100}{12} \text{ J mol}^{-1} \text{K}^{-1}\), and molecular mass of \(O_2 = 32 \text{ g/mol}\), 1 atm pressure = \(1.01 \times 10^5 \text{ N/m}^2\)]
- NEET (UG) - 2025
- Ideal-gas equation and absolute temperature
Concepts Used:
Equilibrium
An equilibrium represents a state in a process when the observable properties such as color, temperature, pressure, concentration etc do not show any change.
The word equilibrium means ‘balance’ which indicates that a chemical reaction represents a balance between the reactants and products taking part in the reaction. The equilibrium state is also noticed in certain physical processes such as the melting point of ice at 0℃, both ice and water are present at equilibrium.
In the case of physical processes such as the melting of solid, dissolution of salt in water etc., the equilibrium is called physical equilibrium while the equilibrium associated with chemical reaction is known as chemical equilibrium.
Equilibrium in Chemical changes
The chemical equilibrium in a reversible reaction is the state at which both forward and backward reactions occur at the same speed.
The stage of the reversible reaction at which the concentration of the reactants and products do not change with time is called the equilibrium state.
Read More: Calculating Equilibrium Concentration
Types of Chemical Equilibrium
There are two types of chemical equilibrium:
- Homogeneous Equilibrium
- Heterogeneous Equilibrium
Homogenous Chemical Equilibrium
In this type, the reactants and the products of chemical equilibrium are all in the same phase. Homogenous equilibrium can be further divided into two types: Reactions in which the number of molecules of the products is equal to the number of molecules of the reactants. For example,
- H2 (g) + I2 (g) ⇌ 2HI (g)
- N2 (g) + O2 (g) ⇌ 2NO (g)
Reactions in which the number of molecules of the products is not equal to the total number of reactant molecules. For example,
- 2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
- COCl2 (g) ⇌ CO (g) + Cl2 (g)
Heterogeneous Chemical Equilibrium
In this type, the reactants and the products of chemical equilibrium are present in different phases. A few examples of heterogeneous equilibrium are listed below.
- CO2 (g) + C (s) ⇌ 2CO (g)
- CaCO3 (s) ⇌ CaO (s) + CO2 (g)
Thus, the different types of chemical equilibrium are based on the phase of the reactants and products.
Check Out: Equilibrium Important Questions
