How many hydrogen bonded water molecule $ (s) $ are associated with $ CuSO_4 \cdot 5H_2O $ ?
- $ 1 $
- $ 2 $
- $ 3 $
- $ 4 $
The Correct Option is A
Solution and Explanation
The structure of complex \(CuSO_{4}, 5H_{2}O\) or \(Cu(H_{2}O)SO_{4} 4H_{2}O\) is as
In crystalline form, four water molecules are coordinated with \(Cu\) atom forming a square-planar geometry and the two \(O\) atoms of sulphate ion complete the distorted octahedron. The fifth water molecule is attached through \(H\)-bonding between one of the coordinated \(H_{2}O\) molecule and one of the sulphate ion
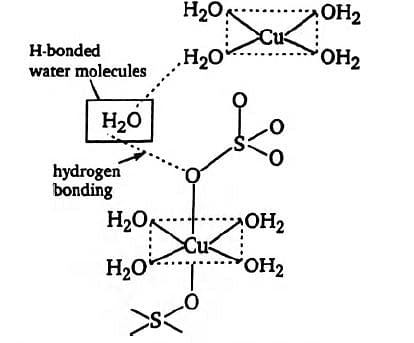
The geometry of copper (II) in copper sulphate pentahydrate is deformed octahedral.
The copper is connected to two oxygen atoms from two sulphate ions and four water molecules in a square-planar geometry, as can be seen in the structure. Another way to put it is that one H2O molecule is H-Bonded to sulphate ions in this situation.
Therefore, four water molecules are in coordination with the Cu2+ ion, while the fifth water molecule is hydrogen-bonded to the oxygen of the sulphate ion. Last but not least, the fifth water molecule is not coordinated, has a hydrogen bond, and is firmly entrenched in a crystal.
Thus, we may say that just four water molecules are coordinated, and the lone hydrogen bond is between the fifth and sixth molecules.
Top Questions on coordination compounds
Given below are two statements regarding conformations of n-butane. Choose the correct option.

- JEE Main - 2026
- Chemistry
- coordination compounds
- The correct statement among the following is:
- JEE Main - 2026
- Chemistry
- coordination compounds
Consider a weak base \(B\) of \(pK_b = 5.699\). \(x\) mL of \(0.02\) M HCl and \(y\) mL of \(0.02\) M weak base \(B\) are mixed to make \(100\) mL of a buffer of pH \(=9\) at \(25^\circ\text{C}\). The values of \(x\) and \(y\) respectively are
- JEE Main - 2026
- Chemistry
- coordination compounds
- \(20.0\,\text{dm}^3\) of an ideal gas \(X\) at \(600\) K and \(0.5\) MPa undergoes isothermal reversible expansion until the pressure of the gas becomes \(0.2\) MPa. Which of the following option is correct? (Given: \(\log 2 = 0.3010\), \(\log 5 = 0.6989\))
- JEE Main - 2026
- Chemistry
- coordination compounds
- From the following : (A) $[\text{Co}(\text{NH}_3)_6]^{3+}$ : Inner orbital complex, $d^2sp^3$ hybridization (B) $[\text{MnCl}_4]^{2-}$ : Outer orbital complex, $sp^3d^2$ hybridization (C) $[\text{CoF}_6]^{3-}$ : Outer orbital complex, $d^2sp^3$ hybridization (D) $[\text{FeF}_6]^{3-}$ : Outer orbital complex, $sp^3d^2$ hybridization (E) $[\text{Ni}(\text{CN})_4]^{2-}$ : Inner orbital complex, $sp^3$ hybridization Choose the correct answer from the given options.
- JEE Main - 2026
- Chemistry
- coordination compounds
Questions Asked in AMUEEE exam
- The ratio of the half-life time $ (t_{1/2}) $ , to the three quarter life-time, $ (t_{3/4}) $ , for a reaction that is second order
- AMUEEE - 2018
- Order of Reaction
- $ 0.002\, M $ solution of a weak acid has an equivalent conductance $ (\wedge) 60\, ohm^{-1}\, cm^{2}\, eq^{-1} $ . What will be the $ pH $ ? (Given : $ \wedge = 400 \, ohm^{-1} \, cm^{2} \, eq^{-1} $ ]
- AMUEEE - 2018
- Electrochemistry
- The electrode potential, $ E^{\circ} $ , for the reduction of $ MnO^{-}_{4} $ to $ Mn^{2+} $ in acidic medium is $ +1.51\, V $ . Which of the following metal(s) will be oxidised? The reduction reactions and standard electrode potentials for $ Zn^{2+}, Ag^{+} $ , and $ Au^{+} $ are given as $ Zn^{2+} (aq) +2e \to Zn(s), E^{\circ} = -0.762\, V $ $ Ag+ (aq) +e {\rightleftharpoons} Ag (s), E^{\circ}=+0.80 \,V $ $ Au^{+} (aq) +e {\rightleftharpoons} Au(s), E^{\circ}+1.69\,V $
- AMUEEE - 2018
- Electrochemistry
- Three particles, two with masses $ m $ and one with mass $ M $ , might be arranged in any of the four configurations shown below. Rank the configurations according to the magnitude of the gravitational force on $ M $ , least to greatest

- AMUEEE - 2018
- Gravitation
- The only force acting on a $ 2 \,kg $ body that is moving in $ xy- $ plane has a magnitude of $ 5\, N $ . The body initially has a velocity of $ 4 \,m/s $ in the positive $ x $ -direction. Some time later, the body has a velocity of $ 6 \,m/s $ in the positive $ y- $ direction. The work done on the body by the $ 5 \,N $ force during this time is
- AMUEEE - 2018
- work, energy and power
Concepts Used:
Coordination Compounds
A coordination compound holds a central metal atom or ion surrounded by various oppositely charged ions or neutral molecules. These molecules or ions are re-bonded to the metal atom or ion by a coordinate bond.
Coordination entity:
A coordination entity composes of a central metal atom or ion bonded to a fixed number of ions or molecules.
Ligands:
A molecule, ion, or group which is bonded to the metal atom or ion in a complex or coordination compound by a coordinate bond is commonly called a ligand. It may be either neutral, positively, or negatively charged.