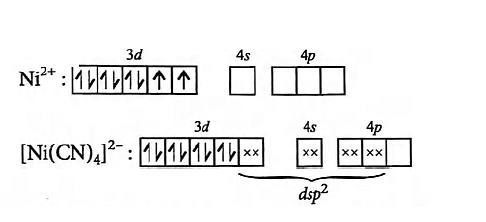
Give reason for the statement. $??Ni(CN)_{4}]^{2-}$ is diamagnetic while $[NiCl_{4}]^{2-}$ is paramagnetic in nature.?
- In $[NiCl_{4}]^{2-}$, no unpaired electrons are present while in $[Ni(CN)_{4}]^{2-}$ two unpaired electrons are present
- In $[Ni(CN)_{4}]^{2-}$, no unpaired electrons are present while in $[NiCl_{4}]^{2-}$ two unpaired electrons are present
- $[NiCl_{4}]^{2-}$ shows $dsp^{2}$ hybridisation hence it is paramagnetic
- $[Ni(CN)_{4}]^{2-}$ shows $sp^{3}$ hybridisation hence it is diamagnetic
The Correct Option is B
Solution and Explanation

Top Questions on coordination compounds
Given below are two statements regarding conformations of n-butane. Choose the correct option.

- JEE Main - 2026
- Chemistry
- coordination compounds
- The correct statement among the following is:
- JEE Main - 2026
- Chemistry
- coordination compounds
Consider a weak base \(B\) of \(pK_b = 5.699\). \(x\) mL of \(0.02\) M HCl and \(y\) mL of \(0.02\) M weak base \(B\) are mixed to make \(100\) mL of a buffer of pH \(=9\) at \(25^\circ\text{C}\). The values of \(x\) and \(y\) respectively are
- JEE Main - 2026
- Chemistry
- coordination compounds
- \(20.0\,\text{dm}^3\) of an ideal gas \(X\) at \(600\) K and \(0.5\) MPa undergoes isothermal reversible expansion until the pressure of the gas becomes \(0.2\) MPa. Which of the following option is correct? (Given: \(\log 2 = 0.3010\), \(\log 5 = 0.6989\))
- JEE Main - 2026
- Chemistry
- coordination compounds
- From the following : (A) $[\text{Co}(\text{NH}_3)_6]^{3+}$ : Inner orbital complex, $d^2sp^3$ hybridization (B) $[\text{MnCl}_4]^{2-}$ : Outer orbital complex, $sp^3d^2$ hybridization (C) $[\text{CoF}_6]^{3-}$ : Outer orbital complex, $d^2sp^3$ hybridization (D) $[\text{FeF}_6]^{3-}$ : Outer orbital complex, $sp^3d^2$ hybridization (E) $[\text{Ni}(\text{CN})_4]^{2-}$ : Inner orbital complex, $sp^3$ hybridization Choose the correct answer from the given options.
- JEE Main - 2026
- Chemistry
- coordination compounds
Concepts Used:
Coordination Compounds
A coordination compound holds a central metal atom or ion surrounded by various oppositely charged ions or neutral molecules. These molecules or ions are re-bonded to the metal atom or ion by a coordinate bond.
Coordination entity:
A coordination entity composes of a central metal atom or ion bonded to a fixed number of ions or molecules.
Ligands:
A molecule, ion, or group which is bonded to the metal atom or ion in a complex or coordination compound by a coordinate bond is commonly called a ligand. It may be either neutral, positively, or negatively charged.