Buna-$N$ synthetic rubber is a copolymer of
Show Hint
Buna-N is unusually high resistance to oils, fuels, and other chemicals and for its superior strength. It's often found in automotive, marine, and aeronautical fuel and oil systems, disposable non-latex gloves, belts, hoses, o-rings and so and so forth. Buna-N is a polymer comprising 2 polymers. This is why it is called a copolymer.

- $H_2C=CH-CH=CH_2$ and $H_5C_6-CH=CH_2$
- $H_2C=CH-CN$ and $H_2C=CH-CH=CH_2$

The Correct Option is C
Solution and Explanation
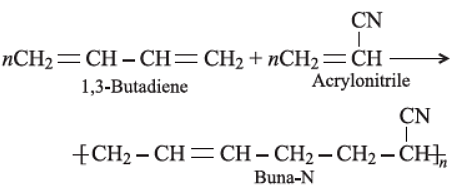
Buna-N is a co-polymer of butadiene and acrylonitrile.
Copolymerisation is a type of polymerisation reaction that involves more than one type of monomer. Buna-N is one such polymer that is formed by copolymerisation. Buna−N is a synthetic rubber copolymer that is made up of 1,3−Butadiene (H2C=CH – CH=CH2) and acrylonitrile (H2C=CH– CN). The synthesis is given below:
Top Questions on Polymers
- A polymer melt being extruded through a spinneret hole of circular cross-section exhibits a die-swell ratio of 2.2. The extrusion velocity and volumetric flow rate during this extrusion process are 20 m/min and \(2.6 \times 10^{-8}\) m\(^3\)/s, respectively. The maximum diameter (mm) of the extruded melt after exit from the spinneret hole (rounded off to 2 decimal places) is:
- The main constituent of vinegar is:
- The monomer of natural rubber is:
- The catalyst used in the preparation of high density polythene is
- Arrange the following polymers in increasing order of intermolecular forces:

Questions Asked in AIEEE exam
- A steel wire can sustain $100\, kg$ weight without breaking. If the wire is cut into two equal parts, each part can sustain a weight of
- AIEEE - 2012
- mechanical properties of solids
- If the line $y = mx + 1$ meets the circle $x^2 + y^2 + 3x = 0 $ in two points equidistant from and on opposite sides of $x$-axis, then
- AIEEE - 2012
- Conic sections
- This question has Statement 1 and Statement 2. Of the four choices given after the Statements, choose the one that best describes the two Statements. If you push on a cart being pulled by a horse so that it does not move, the cart pushes you back with an equal and opposite force. The cart does not move because the force described in statement 1 cancel each other.
- AIEEE - 2012
- potential energy
- The variance of first n odd natural numbers is $\frac{n^{2}-1}{3}$ : The sum of first n odd natural number is $n^2$ and the sum of square of first n odd natural numbers is $\frac{n\left(4n^{2}-1\right)}{3}.$
- AIEEE - 2012
- Variance and Standard Deviation
- The ratio of number of oxygen atoms (O) in 16.0 g ozone $(O_3), \,28.0\, g$ carbon monoxide $(CO)$ and $16.0$ oxygen $(O_2)$ is (Atomic mass: $C = 12,0 = 16$ and Avogadro?? constant $N_A = 6.0 x 10^{23}\, mol^{-1}$)
- AIEEE - 2012
- Mole concept and Molar Masses
Concepts Used:
Biodegradable Polymers
Microorganisms destroy biodegradable polymers in an appropriate amount of time, ensuring that biodegradable polymers and their degraded products have a low environmental result. Enzyme-catalyzed processes shatter these polymers down into little segments, and microorganisms manufacture these enzymes.
Biodegradable Polymers: Examples
- Poly-β-hydroxybutyrate-co-β-hydroxy valerate (PHBV)
- Polylactic Acid (PLA)
- Poly (ε-caprolactone) (PCL)
- Nylon-2-Nylon-6
Biodegradable Polymers: Properties
- Can maintain strong mechanical unification until they are degraded.
- Degradation, in general, starts at the end groups because biodegradable polymers have exceptionally strong carbon backbones that are difficult to crack.
- Non-toxic in nature.
- Degradation rates can be controlled.
- Lack of crystallinity, which inhibits access to end groups.
- Hydrophilic polymers.
Read More: Biopolymers