An organic compound exhibits the [M]+, [M+2]+, and [M+4]+ peaks in the intensity ratio 1:2:1 in the mass spectrum, and shows a singlet at \( \delta \) 7.49 in the \( ^1H \) NMR spectrum in CDCl\(_3\). The compound is:
Show Hint
- 1,4-dichlorobenzene
- 1,4-dibromobenzene
- 1,2-dibromobenzene
- 1,2-dichlorobenzene
The Correct Option is B
Solution and Explanation
Step 1: Analyze the Mass Spectrum.
The given intensity ratio 1:2:1 for the [M]+, [M+2]+, and [M+4]+ peaks suggests that the compound contains a halogen, which leads to the isotopic pattern due to the presence of isotopes. The 2:1 ratio is characteristic of a bromine atom, as bromine has two naturally occurring isotopes: \( ^{79} \text{Br} \) and \( ^{81} \text{Br} \), which give this ratio in the mass spectrum.
Step 2: Analyze the NMR Spectrum.
A singlet at \( \delta = 7.49 \) in the \( ^1H \) NMR spectrum suggests a symmetrical environment for the protons, which is consistent with the 1,4-substitution pattern in the benzene ring.
Step 3: Conclusion.
Based on the mass spectrum and the NMR singlet, the compound is most likely 1,4-dibromobenzene.
Final Answer: \[ \boxed{\text{1,4-dibromobenzene}} \]
Top Questions on Chirality & symmetry of organic molecules with or without chiral centres
- Which of the following molecules is chiral in nature?
- CBSE CLASS XII - 2025
- Chemistry
- Chirality & symmetry of organic molecules with or without chiral centres
- In the given molecule,

the number of chiral centers is _______.- IIT JAM BT - 2024
- Chemistry
- Chirality & symmetry of organic molecules with or without chiral centres
- The correct statement(s) about the relationship for the H-atoms in the following compounds is (are) :
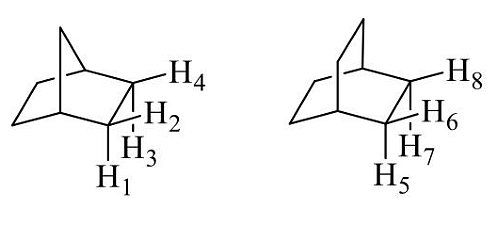
- GATE CY - 2024
- Organic Chemistry
- Chirality & symmetry of organic molecules with or without chiral centres
- Among the following, the chiral compound is
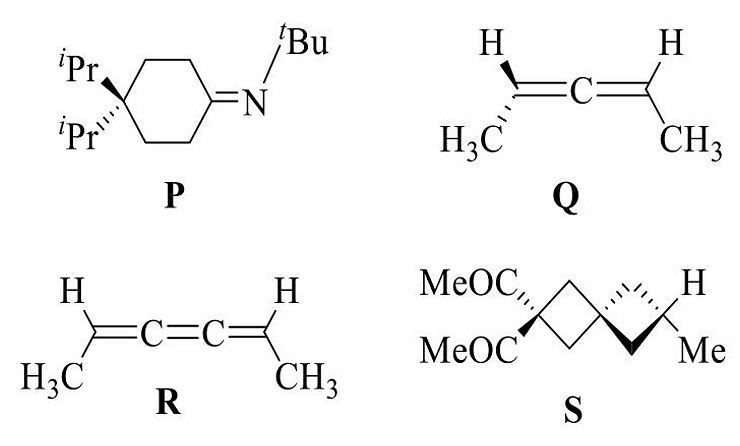
- GATE CY - 2024
- Organic Chemistry
- Chirality & symmetry of organic molecules with or without chiral centres
Which one of the following compounds is the simplest alkane that is optically active?

- IIT JAM BT - 2020
- Chemistry
- Chirality & symmetry of organic molecules with or without chiral centres
Questions Asked in GATE CY exam
- For a first-order reaction, the unit of rate constant is:
- GATE CY - 2026
- Chemical Kinetics
- Which ligand causes maximum crystal field splitting?
- GATE CY - 2026
- Coordination chemistry
- The standard electrode potential of the standard hydrogen electrode (SHE) is:
- GATE CY - 2026
- Electrochemistry
- Which of the following reagents converts an aldehyde selectively into a primary alcohol?
- GATE CY - 2026
- Organic Chemistry
- In IR spectroscopy, which bond absorbs at the highest wavenumber?
- GATE CY - 2026
- Spectroscopy