A precipitate is formed if the ionic product is __________?
A precipitate is formed if the ionic product is __________?
Greater than the solubility product
Lesser than the solubility product
Equal to solubility product
Independent of solubility product
The Correct Option is A
Solution and Explanation
In chemistry, the formation of a precipitate is related to the concept of solubility product (Ksp). The solubility product is a constant for a given substance at a specific temperature, representing the maximum product of ion concentrations in a saturated solution. The ionic product is the product of the concentrations of ions in a solution raised to the power of their stoichiometric coefficients in the balanced equation of dissolution.
A precipitate forms when the ionic product exceeds the solubility product (Ksp), as this indicates that the solution is supersaturated with respect to the dissolved ions. Supersaturation leads to excess ions pairing to form an insoluble solid, i.e., a precipitate, because the solution can no longer maintain such high ion concentrations in the dissolved state.
Therefore, a precipitate is formed if the ionic product is greater than the solubility product.
Top Questions on Law Of Chemical Equilibrium And Equilibrium Constant
- Consider the following gaseous equilibrium in a closed container of volume $V$ at temperature $T$:
P$_2$(g) + Q$_2$(g) $\rightleftharpoons$ 2PQ(g)
Initially, 2 moles each of P$_2$(g), Q$_2$(g) and PQ(g) are present at equilibrium. One mole each of P$_2$ and Q$_2$ are added. The number of moles of P$_2$, Q$_2$ and PQ at the new equilibrium respectively are
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- $X_2(g) + Y_2(g) \rightleftharpoons 2Z(g)$. Equilibrium moles of $X_2, Y_2, Z$ are 3, 3, 9 mol (in 1 L). 10 mol of Z(g) is added. New equilibrium moles of Z(g) is ___. (Nearest integer)
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- Observe the following equilibrium in a 1 L flask:
A(g) <=> B(g)
At temperature T (in K), the equilibrium concentrations of A and B are 0.5 M and 0.375 M respectively.
Then 0.1 moles of A is added into the flask and the system is heated to temperature T again to re-establish equilibrium.
The new equilibrium concentrations (in M) of A and B respectively are:- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- The plot of \(\log_{10}K\) vs \(\frac{1}{T}\) gives a straight line. The intercept and slope respectively are (where \(K\) is equilibrium constant).
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- For the reaction \( \mathrm{A \rightleftharpoons B} \), the number of moles of A and B at equilibrium in a \(1\,\text{L}\) vessel are \(0.50\) and \(0.375\), respectively. If \(0.10\) mol of A is added further, determine the number of moles of A and B at the new equilibrium.
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
Questions Asked in SRMJEEE exam
- Markovnikov's rule is applicable to which of the following reactions?
- SRMJEEE - 2025
- Chemical Reactions
- Calculate the Reynolds number for a liquid with density 1 g/cm³ and viscosity 8 × 10⁻⁴ Pa·s, flowing at 0.5 m/s through a pipe of diameter 4 cm.
- SRMJEEE - 2025
- Viscosity
- A current of 2 A passes through a coil of 100 turns, producing a magnetic flux of 5 × 10⁻⁵ Wb per turn. What is the magnetic energy associated with the coil?
- SRMJEEE - 2025
- Magnetic Field
- A box contains 5 red balls and 7 blue balls. Two balls are drawn at random without replacement. What is the probability that both balls are red?
- SRMJEEE - 2025
- Probability
- The molar conductivities at infinite dilution for Na₂SO₄, K₂SO₄, KCl, HCl, and HCOONa at 300 K are 260, 308, 150, 426, and 105 S·cm²·mol⁻¹, respectively. What will be the molar conductivity at infinite dilution (Λ⁰) for formic acid in the same unit?
- SRMJEEE - 2025
- Mole concept and Molar Masses
Concepts Used:
Law of Chemical Equilibrium
Law of Chemical Equilibrium states that at a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation.
Let us consider a general reversible reaction;
A+B ↔ C+D
After some time, there is a reduction in reactants A and B and an accumulation of the products C and D. As a result, the rate of the forward reaction decreases and that of backward reaction increases.
Eventually, the two reactions occur at the same rate and a state of equilibrium is attained.
By applying the Law of Mass Action;
The rate of forward reaction;
Rf = Kf [A]a [B]b
The rate of backward reaction;
Rb = Kb [C]c [D]d
Where,
[A], [B], [C] and [D] are the concentrations of A, B, C and D at equilibrium respectively.
a, b, c, and d are the stoichiometric coefficients of A, B, C and D respectively.
Kf and Kb are the rate constants of forward and backward reactions.
However, at equilibrium,
Rate of forward reaction = Rate of backward reaction.
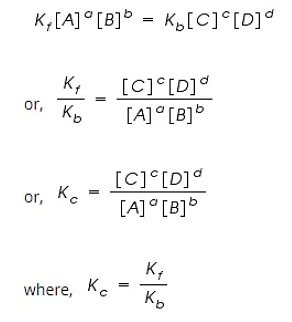
Kc is called the equilibrium constant expressed in terms of molar concentrations.
The above equation is known as the equation of Law of Chemical Equilibrium.