1 mole of HI is heated in a closed container of capacity of 2 L. At equilibrium half a mole of HI is dissociated. The equilibrium constant of the reaction is
1 mole of HI is heated in a closed container of capacity of 2 L. At equilibrium half a mole of HI is dissociated. The equilibrium constant of the reaction is
- 0.25
- 1
- 0.35
- 0.5
The Correct Option is A
Approach Solution - 1
Correct Answer: 0.25
Explanation:
The reaction is: 2HI ⇌ H2 + I2
Initially: HI = 1 mole, H2 = 0, I2 = 0
At equilibrium (since half a mole of HI is dissociated): → HI dissociates = 0.5 mol → 0.5 mol HI gives 0.25 mol H2 and 0.25 mol I2 Remaining HI = 1 − 0.5 = 0.5 mol
Now using equilibrium concentrations (in mol/L): Volume = 2 L [HI] = 0.5 / 2 = 0.25 mol/L [H2] = 0.25 / 2 = 0.125 mol/L [I2] = 0.25 / 2 = 0.125 mol/L
Equilibrium constant (Kc): \[ K_c = \frac{[H_2][I_2]}{[HI]^2} = \frac{0.125 \times 0.125}{(0.25)^2} = \frac{0.015625}{0.0625} = 0.25 \]
Approach Solution -2
The dissociation of HI is given by the balanced equation: \[ 2HI \rightleftharpoons H_2 + I_2 \] The equilibrium constant expression for this reaction is: \[ K_c = \frac{[H_2] \cdot [I_2]}{[HI]^2} \] We are given that at equilibrium, half a mole of HI dissociates in a 2 L container. So, the equilibrium concentrations are as follows: \[ [HI] = \frac{0.5 \, \text{mole}}{2 \, \text{L}} = 0.25 \, \text{M} \] Since half of the HI dissociates, the concentrations of \(H_2\) and \(I_2\) will each be 0.125 M: \[ [H_2] = 0.5 \times 0.25 = 0.125 \, \text{M} \] \[ [I_2] = 0.5 \times 0.25 = 0.125 \, \text{M} \] Substituting these values into the equilibrium constant expression: \[ K_c = \frac{(0.125) \cdot (0.125)}{(0.25)^2} = \frac{0.015625}{0.0625} = 0.25 \] Thus, the equilibrium constant of the reaction is \(K_c = 0.25\). Therefore, the correct_
Top Questions on Law Of Chemical Equilibrium And Equilibrium Constant
- Consider the following gaseous equilibrium in a closed container of volume $V$ at temperature $T$:
P$_2$(g) + Q$_2$(g) $\rightleftharpoons$ 2PQ(g)
Initially, 2 moles each of P$_2$(g), Q$_2$(g) and PQ(g) are present at equilibrium. One mole each of P$_2$ and Q$_2$ are added. The number of moles of P$_2$, Q$_2$ and PQ at the new equilibrium respectively are
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- $X_2(g) + Y_2(g) \rightleftharpoons 2Z(g)$. Equilibrium moles of $X_2, Y_2, Z$ are 3, 3, 9 mol (in 1 L). 10 mol of Z(g) is added. New equilibrium moles of Z(g) is ___. (Nearest integer)
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- Observe the following equilibrium in a 1 L flask:
A(g) <=> B(g)
At temperature T (in K), the equilibrium concentrations of A and B are 0.5 M and 0.375 M respectively.
Then 0.1 moles of A is added into the flask and the system is heated to temperature T again to re-establish equilibrium.
The new equilibrium concentrations (in M) of A and B respectively are:- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- The plot of \(\log_{10}K\) vs \(\frac{1}{T}\) gives a straight line. The intercept and slope respectively are (where \(K\) is equilibrium constant).
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- For the reaction \( \mathrm{A \rightleftharpoons B} \), the number of moles of A and B at equilibrium in a \(1\,\text{L}\) vessel are \(0.50\) and \(0.375\), respectively. If \(0.10\) mol of A is added further, determine the number of moles of A and B at the new equilibrium.
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
Questions Asked in KCET exam
Match the following:
In the following, \( [x] \) denotes the greatest integer less than or equal to \( x \).
Choose the correct answer from the options given below:- KCET - 2025
- Differentiability
- If \[ y = \frac{\cos x}{1 + \sin x} \] then:
- KCET - 2025
- Differentiability
- A function \( f(x) \) is given by:
\[ f(x) = \begin{cases} \frac{1}{e^x - 1}, & \text{if } x \neq 0 \\ \frac{1}{e^x + 1}, & \text{if } x = 0 \end{cases} \] Then, which of the following is true?- KCET - 2025
- Limits
- The function f(x) is given by:
For x < 0:
f(x) = ex + axFor x ≥ 0:
f(x) = b(x - 1)2
The function is differentiable at x = 0. Then,- KCET - 2025
- Differentiability
- The function \( f(x) = \tan x - x \)
- KCET - 2025
- Derivatives
Concepts Used:
Law of Chemical Equilibrium
Law of Chemical Equilibrium states that at a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation.
Let us consider a general reversible reaction;
A+B ↔ C+D
After some time, there is a reduction in reactants A and B and an accumulation of the products C and D. As a result, the rate of the forward reaction decreases and that of backward reaction increases.
Eventually, the two reactions occur at the same rate and a state of equilibrium is attained.
By applying the Law of Mass Action;
The rate of forward reaction;
Rf = Kf [A]a [B]b
The rate of backward reaction;
Rb = Kb [C]c [D]d
Where,
[A], [B], [C] and [D] are the concentrations of A, B, C and D at equilibrium respectively.
a, b, c, and d are the stoichiometric coefficients of A, B, C and D respectively.
Kf and Kb are the rate constants of forward and backward reactions.
However, at equilibrium,
Rate of forward reaction = Rate of backward reaction.
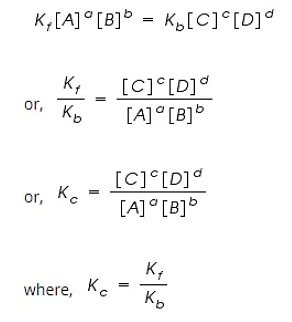
Kc is called the equilibrium constant expressed in terms of molar concentrations.
The above equation is known as the equation of Law of Chemical Equilibrium.